Ventus Service

Engineering and Design Resources The process design engineers at VENTUS PROCESS offer several services including industrial process design, hygienic process design, Clean-ln Place (CIP) system design and circuitry, and piping design. Process design engineers at VENTUS PROCESS work with you to evaluate your uniqe needs and assume responsibiliry for the complex details of multi-phase […]
Large Diameter Pipe and Duct Fabrication

We are able to fabricate insulated/uninsulated large diameters ducts and pipes. All stainless steel, with the requested surface hardness.
Engineering, Validation, Automation
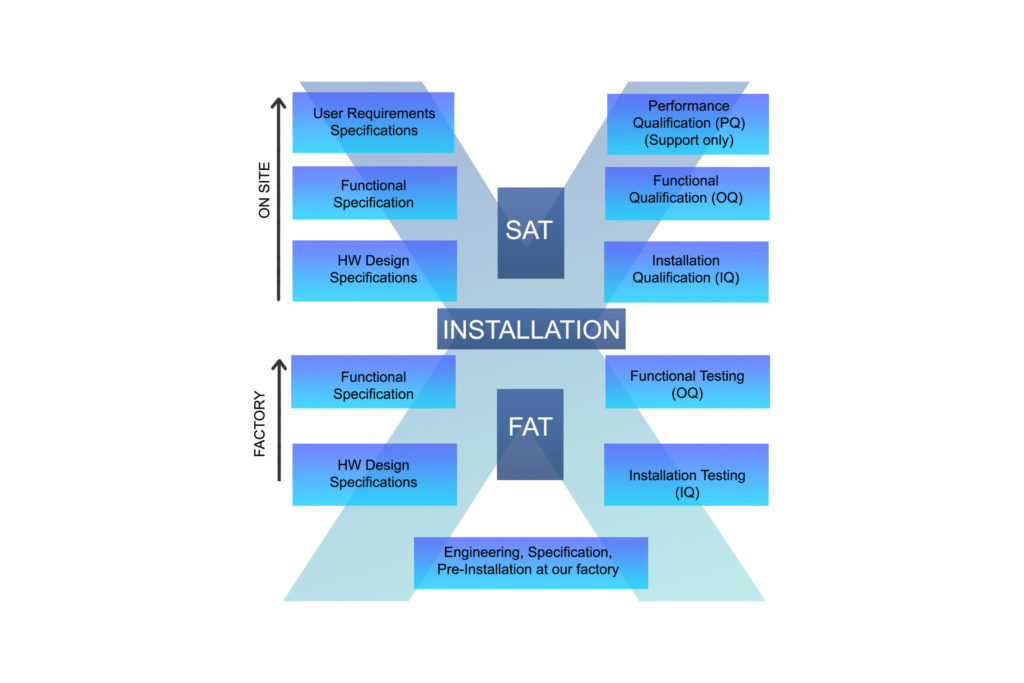
User Requirement Specification Validation Plan Quality Plan Functional Specification Sequences/Recipes HW Design Specifications SW (Module Design Specifications) PLC/OPC/Feldbus/SCADAVLAN HW Construction HW Purchase SW Implementation HW Component Acceptance SW Module Testing(IQ Protocol and Report) HW Integration Test SW Integration Test (OQ Protocol and Report) Validation Report System Acceptance (Support only) User Requirements Specifications Functional Specification HW […]
Fabrication

We produce out of standards! What you need for your system. Great and certificated welding and assembly teams of Ventus Process offer you to fabricate any kind of out of standard part you need for your process.
Bio-Pharma Vaccine

Bio-pharma and vaccine industries require large investment in time and capital to effectively translate scientific discovery into novel and affordable therapies. With years of experience, VENTUS PROCESS has become a strategic driver in providing scalable and flexible bioprocess solutions of high quality. Efficient Systems: In-Situ Fermenters/ Bioreactors Upstream bioprocess vessels for Media /Buffer preparation, Feed […]
Sterile Formulations
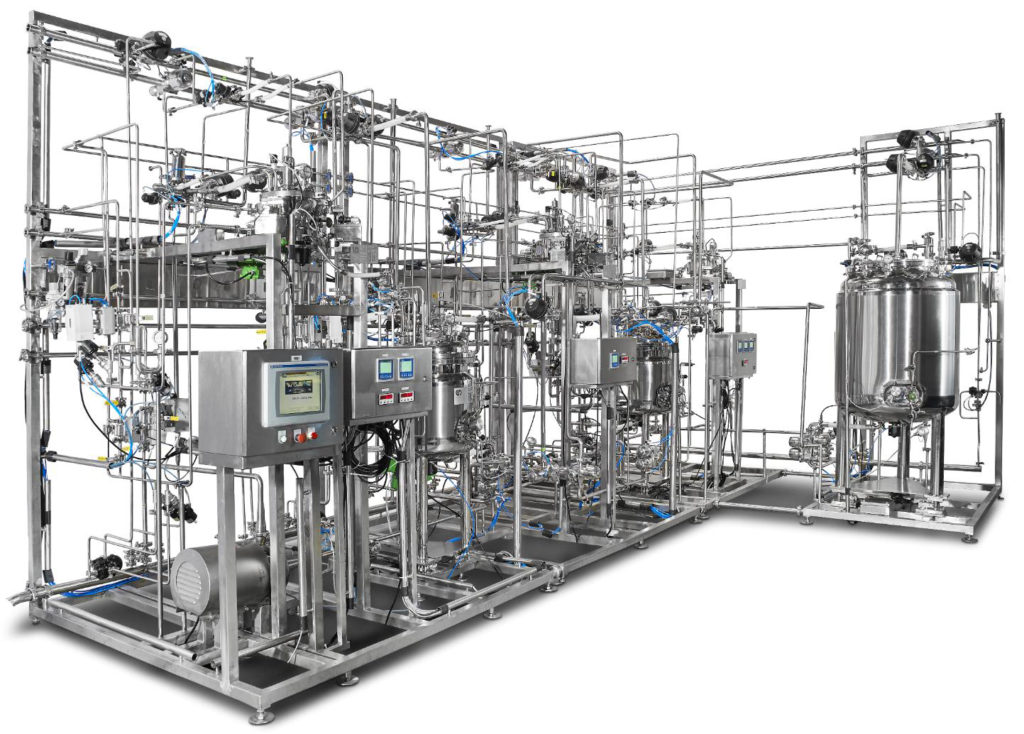
Sterile formulations manufacturers are looking for strong and effective solutions to compound a wide variety of formulations, which include products administered by injection (IV, IM, IP, SC-SQ, ID, intrathecal, epidural) or via otic, intranasal or ophthalmic routes. We have decades of experience in developing innovative technologies and smarter solutions for challenging pyrogen free and sterile […]
Manufacturing System-MDI-DPI

There has been a significant rise in theprevalence of the diseases that are related to the respiratory system such as chronic obstructive pulmonary diseases, asthma, chronic respiratory diseases and others. The demand for the metered dose inhalers, Dry powder inhalation and nebulizers has increased. We have vast years of experience in designing and executing standalone […]
Distillation (WFI) Unit

Distillation (WFI) Unit Design Total energy saving is 75% as less heating energy and less cooling water are required. Pyrogens are removed by centrifugai force developed & specially designed spiral baffle. All contact parts are SS316 quality. All pipes and tubes are seamless, and PTFE gaskets. Distillate Temperature is quite high; 90 C – 95C […]
Purified Water(PW)-Water for Injection(WFI)

WATER, A BASIC ELEMENT FOR PRODUCTION Purified Water and Water for Injection Production VENTUS PROCESS design that Reverse Osmosis + Electro Deionization (RO + EDI) plants constitute a reliable alternative to the traditional double stage Reverse Osmosis for the production purified water. VENTUS PROCESS design RO+EDI plants in compliance with the standards requested by International […]
CIP-WIP-SIP

EFFICIENT SANIT ARY AND INDUS TRIAL CLEAN – IN – PLACE (CIP) SYSTEMS From design and fabrication to installation and start up by Ventus Process. CIP/SIP/WIP skids are designed to clean and/or sterilize eqipment in pharmaceutical, biotechnological, cosmetic and food & beverage industry production, guaranteeing compliance with the strict cleanliness re@irements of these sectors. We […]
